The path to over-the-counter (OTC) hearing aids was recently discussed in a presentation held by Thomas A. Powers, PhD on Audiology Online. He provided an in-depth overview of the pathway from the early PCAST through the regulation, the FDA, and how all that worked. I wanted to summarize it today.
Perceptions that Drove the Creation of OTC Hearing Aids
One of the main drivers behind the creation of the OTC category of hearing aids by the FDA was cost. The issue of cost has always been a concern, whether it’s on the prescription side or the OTC side. Access to a professional was also a significant factor that was discussed in the early meetings held by PCAST, NASM, FDA, FTC, and a growing body of research and awareness around associated comorbidities.
One of the most commonly mentioned comorbidities related to hearing loss is cognitive decline, including dementia and Alzheimer’s disease. Treating hearing loss has been identified as one of the modifiable risks for cognitive decline, and this has generated a lot of interest. However, it has also led to some misperceptions and misinformation in the marketplace.

The Lack of Coverage within Medicare
Since 1965, the original Medicare statutes excluded hearing aids and hearing aid-related services from coverage, as well as dental and vision. Back then, there wasn’t enough information about the specific benefits of hearing aids related to communication needs, both partners, work, play, etc. Coverage is limited to some degree, and what coverage there is for hearing imbalance exams, cochlear implementation, and osteointegration devices for those who meet those criteria. With limited coverage, cost becomes a significant barrier to accessing hearing aids.
Insurance coverage is also somewhat limited, but we are seeing emerging access through Medicare Advantage plans. These plans include hearing, vision, and dental to some degree, which is attractive to those looking to gain membership in those plans because these are healthcare issues that are not covered under Medicare at the present time.
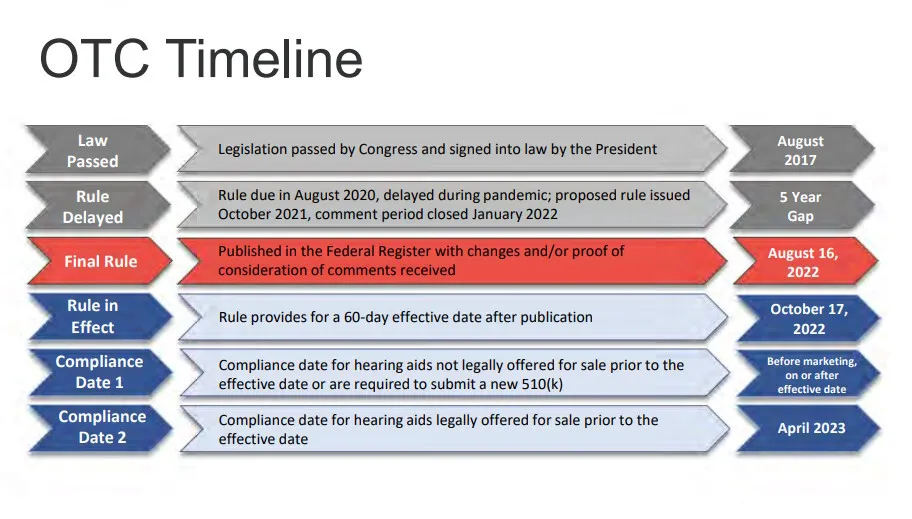
The Timeline for OTC Hearing Aids
The path to OTC hearing aids started with various workshops held by PCAST, National Academy of Science, Engineering and Medicine, FDA, FTC, and others. Originally, an NIH meeting in 2009 was the impetus behind the access and affordability issue that drove the OTC regulations. The regulations were passed in August 2017, and due to the pandemic and other factors within the FDA, it took almost five years to get the proposed rule. The final rule was published in August, and the effective date was October 17th, 2022, which allowed the sale of OTC devices and their variety of farm factors to be available to the public.
Compliance Dates for Existing and New Companies
Two critical parts of the implementation of OTC hearing aids are the compliance dates for both the existing companies as well as new companies. For hearing aids not legally offered prior to the effective date, they are required to submit new 510s before they could start that, while they were looking at the rule and understanding what the rule was. For those products that were legally offered before the effective date, they have a compliance date of April 2023, which means those devices that were on the market and had already applied to the FDA for their approval or release to market have until April to comply with the new regulations.